- About Us
- Company
- Chairman’s Message
- Team
- Course
- Corporate Culture
- Honour
- Technology
- Research and Develop
- Innovation Platform
- Research and Development Pipeline
- Innovative Drugs
- Product
- Digestive System
- Cardiovascular System
- Endocrine System
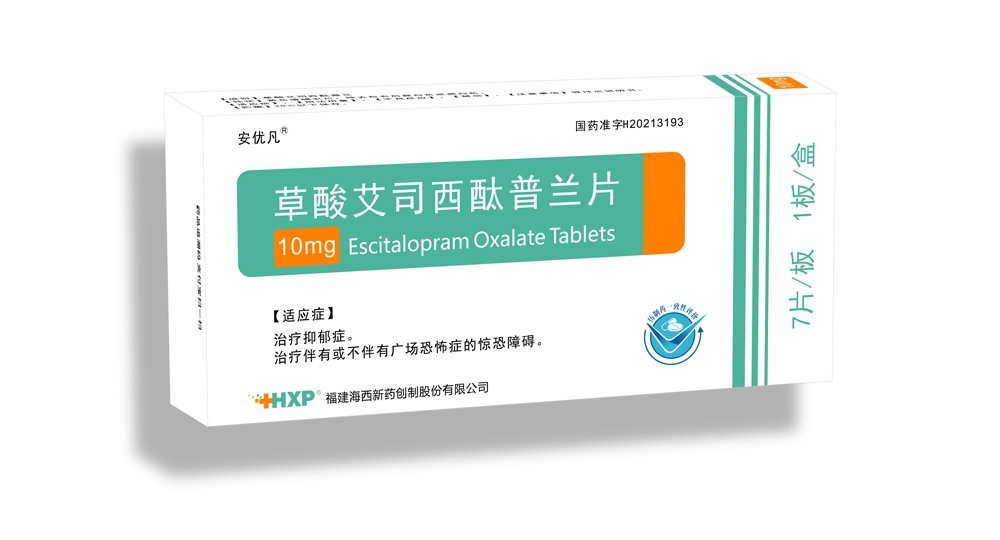
- Nervous System
- Inflammation
- Respiratory System
- News
- Company News
- Media Coverage
- Investor
- Announcements and Circulars
- Financial Reports
- Prospectus
- Corporate Governance
- Contact
- Attract Talents
- Talent Development
- Recruitment Position
- Contact Us
- Contact Us
- Product Consultation